Can Vitamin C Increase Appetite
Contents
- Summary
- Function
- Visual system and eyesight
- Regulation of gene expression
- Immunity
- Prenatal and postnatal development
- Red blood cell production
- Nutrient interactions
- Deficiency
- Vitamin A deficiency-related disorders
- The RDA
- Retinol activity equivalents (RAE)
- Determination
- Disease Prevention
- Bronchopulmonary dysplasia in preterm infants
- Childhood morbidity and mortality
- Cancer
- Disease Treatment
- Acute promyelocytic leukemia
- Diseases of the skin
- Retinitis pigmentosa
- Sources
- Food
- Supplements
- Safety
- Toxicity
- Pregnancy
- Osteoporosis risk
- Drug interactions
- LPI Recommendation
- Authors and Reviewers
- References
Español | 日本語
Summary
- Vitamin A is a generic term that refers to fat-soluble compounds found as preformed vitamin A (retinol) in animal products and as provitamin A carotenoids in fruit and vegetables. The three active forms of vitamin A in the body are retinol, retinal, and retinoic acid. (More information)
- Vitamin A is involved in regulating the growth and specialization (differentiation) of virtually all cells in the human body. Vitamin A has important roles in embryonic development, organ formation during fetal development, normal immune functions, and eye development and vision. (More information)
- Vitamin A deficiency is a major cause of preventable blindness in the world. It is most prevalent among children and women of childbearing age. Vitamin A deficiency is associated with an increased susceptibility to infections, as well as to thyroid and skin disorders. (More information)
- The recommended dietary allowance (RDA) is 700 micrograms of retinol activity equivalents (μg RAE)/day for women and 900 μg RAE/day for men. (More information)
- Vitamin A prophylaxis appears to significantly reduce childhood mortality in regions at high risk of vitamin A deficiency. Further, high-dose vitamin A supplementation is widely recommended for children over six months of age when they are infected with measles while malnourished, immunodeficient, or are at risk of measles complications. (More information)
- Retinoic acid and analogs are used at pharmacological doses in the treatment of acute promyelocytic leukemia and various skin diseases. (More information)
- Animal food sources rich in preformed vitamin A include dairy products, fortified cereal, liver, and fish oils. Rich sources of provitamin A carotenoids include orange and green vegetables, such as sweet potato and spinach. (More information)
- Overconsumption of preformed vitamin A can be highly toxic and is especially contraindicated prior to and during pregnancy as it can result in severe birth defects. The tolerable upper intake level (UL) for vitamin A in adults is set at 3,000 μg RAE/day. The UL does not apply to vitamin A derived from carotenoids. (More information)
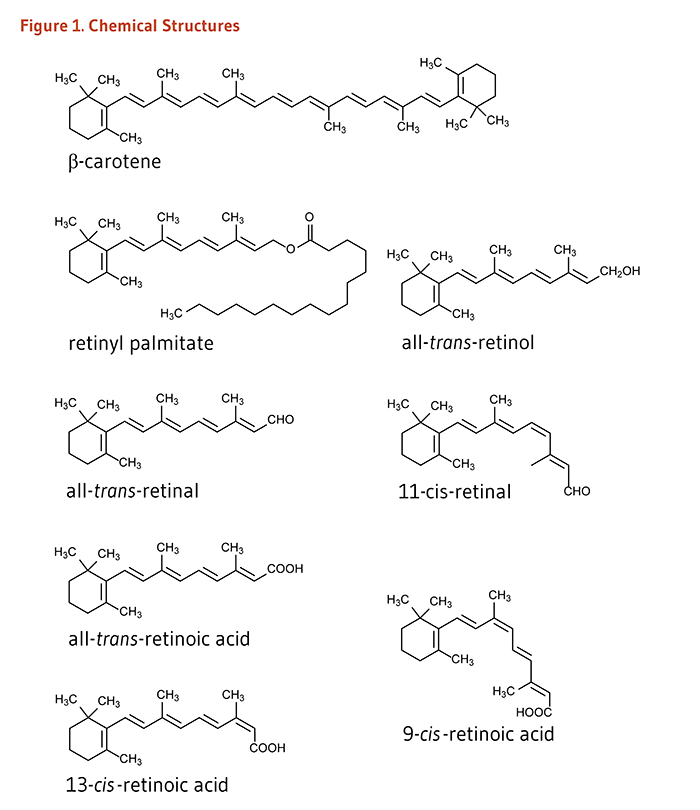
Vitamin A is a generic term that encompasses a number of related compounds (Figure 1). Retinol and retinyl esters are often referred to as preformed vitamin A. Retinol can be converted by the body to retinal, which can be in turn be oxidized to retinoic acid, the form of vitamin A known to regulate gene transcription. Retinol, retinal, retinoic acid, and related compounds are known as retinoids. β-Carotene and other food carotenoids that can be converted by the body into retinol are referred to as provitamin A carotenoids (see the article on Carotenoids). Hundreds of different carotenoids are synthesized by plants, but only about 10% of them are capable of being converted to retinol (1). The following discussion will focus mainly on preformed vitamin A compounds and retinoic acid.
Function
Vitamin A compounds are essential fat-soluble molecules predominantly stored in the liver in the form of retinyl esters (e.g., retinyl palmitate). When appropriate, retinyl esters are hydrolyzed to generate all-trans-retinol, which binds to retinol binding protein (RBP) before being released in the bloodstream. The all-trans-retinol/RBP complex circulates bound to the protein, transthyretin, which delivers all-trans-retinol to peripheral tissues (reviewed in 2). Vitamin A as retinyl esters in chylomicrons was also found to have an appreciable role in delivering vitamin A to extrahepatic tissues, especially in early life (3, 4).
Visual system and eyesight
Located at the back of the eye, the retina contains two main types of light-sensitive receptor cells − known as rod and cone photoreceptor cells. Photons (particles of light) that pass through the lens are sensed by the photoreceptor cells of the retina and converted to nerve impulses (electric signals) for interpretation by the brain. All-trans-retinol is transported to the retina via the circulation and accumulates in retinal pigment epithelial (RPE) cells (Figure 2) (5). Here, all-trans-retinol is esterified to form a retinyl ester, which can be stored. When needed, retinyl esters are broken apart (hydrolyzed) and isomerized to form 11-cis-retinol, which can be oxidized to form 11-cis-retinal. 11-cis-retinal can be shuttled across the interphotoreceptor space to the rod photoreceptor cell that is specialized for vision in low-light conditions and for detection of motion. In rod cells, 11-cis-retinal binds to a protein called opsin to form the visual pigment rhodopsin (also known as visual purple). Absorption of a photon of light catalyzes the isomerization of 11-cis-retinal to all-trans-retinal that is released from the opsin molecule. This photoisomerization triggers a cascade of events, leading to the generation of a nerve impulse conveyed by the optic nerve to the brain's visual cortex. All-trans-retinal is converted to all-trans-retinol and transported across the interstitial space to the RPE cells, thereby completing the visual cycle.
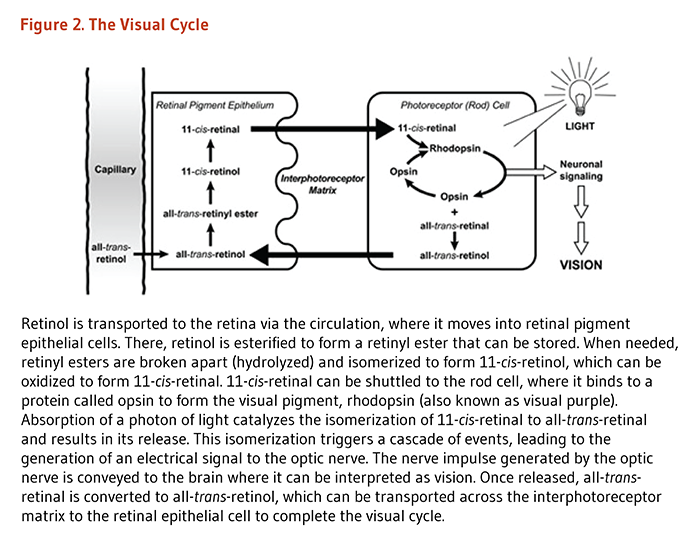
A similar cycle occurs in cone cells that contain red, green, or blue opsin proteins required for the absorption of photons from the visible light spectrum (2). Vitamin A is also essential for mammalian eye development (6). Thus, because vitamin A is required for the normal functioning of the retina, dim-light vision, and color vision, inadequate retinol and retinal available to the retina result in impaired dark adaptation. In the severest cases of vitamin A deficiency, thinning and ulceration of the cornea leads to blindness (see Deficiency).
Regulation of gene expression
Regulatory capacity of retinoic acid
In cells, all-trans-retinol can be either stored (in the form of retinyl ester) or oxidized to all-trans-retinal by alcohol dehydrogenases. In turn, retinaldehyde dehydrogenases can catalyze the conversion of all-trans-retinal into two biologically active isomers of retinoic acid (RA): all-trans-RA and 9-cis-RA. RA isomers act as hormones to affect gene expression and thereby influence numerous physiological processes. All-trans-RA and 9-cis-RA are transported to the nucleus of the cell bound to cellular retinoic acid-binding proteins (CRABP). Within the nucleus, RA isomers bind to specific nuclear receptor proteins that are ligand-dependent transcription factors (Figure 3). Both all-trans-RA and 9-cis-RA can bind to retinoic acid receptors (RARα, RARβ, and RARγ), whereas only 9-cis-RA binds to retinoid X receptors (RXRα, RXRβ, and RXRβ) (7). RAR and RXR subtypes form either complexes of two of the same protein (RAR/RAR and RXR/RXR homodimers) or complexes of two different proteins (RAR/RXR heterodimers). RAR/RXR heterodimers can bind to a regulatory DNA sequence called retinoic acid response element (RARE) located within the promoter of retinoid-responsive genes. The transcriptional activity of RAR/RXR heterodimers appears to be mainly driven by the binding of all-trans-RA to RAR.
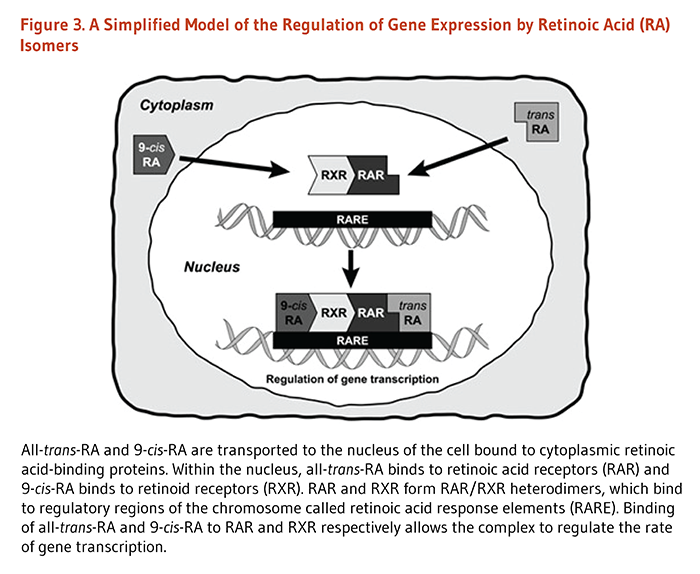
The activation of RAR by RA binding triggers the recruitment of transcriptional coregulators to target promoters, thereby inhibiting or allowing the transcription of genes (8). RXR also forms heterodimers with several other nuclear receptors, including thyroid hormone receptor (TR), vitamin D receptor (VDR), steroid receptors, and peroxisome proliferator-activated receptor (PPAR) (9). In this way, vitamin A may interact with thyroid hormone, vitamin D, steroids (e.g., estrogen), or PPAR ligands signaling pathways and influence the transcription of a broad range of genes.
There is also evidence that RA/RAR can affect gene expression in a RARE-independent manner. For example, it was reported that RAR could interfere with TGFβ/Smad signaling pathway through direct interaction of RAR with the heterodimeric transcription factor, Smad3/Smad4. In the absence of RA, RAR was found to act as a coactivator of Smad3/Smad4-mediated transcription, while RAR agonists repressed the transcriptional activity of Smad3/Smad4 (10). In retinoblastoma cells, RAR was also involved in RA-induced activation of signaling cascades mediated by tyrosine kinases known as phosphoinositide 3-kinase (PI3K) and leading to cell differentiation (11, 12). RA also appeared to induce neuronal differentiation by activating ERK1/2 MAP kinase signaling pathway that phosphorylated transcription factor, CREB (cyclic AMP response element binding protein). Phosphorylated CREB can subsequently bind to the CREB response element in the promoter of genes involved in cell differentiation (13). Also, independently of RAR, RA was found to inhibit ERK1/2 phosphorylation/activation and subsequent AP1-mediated expression of interleukin-6 in synovial cells (14). Hence, RA can influence the expression of genes whose promoters do not contain RARE.
By regulating the expression of over 500 retinoid-responsive genes (including several genes involved in vitamin A metabolism itself), retinoic acid isomers play major roles in cellular proliferation and differentiation (i.e., cell commitment to highly specialized functions).
Regulatory capacity of retinol
In the eye and tissues like white adipose and muscle, retinol plasma membrane receptor/transporter STRA6 accepts retinol from extracellular RBP and unloads it to intracellular retinol-binding protein (CRBP). STRA6 also cooperates with lecithin:retinol acyltransferase (LRAT), an enzyme that catalyzes retinol esterification and storage, to maintain an inward concentration gradient of retinol (15). Interestingly, retinol uptake by STRA6 was found to trigger the activation of a signaling cascade mediated by tyrosine kinases known as Janus kinases (JAK) and associated transcription factors (STAT). JAK/STAT signaling pathway regulates the expression of a wide range of cytokines, hormones, and growth factors (16). Animal studies have reported that an increased expression of genes, such as SOCS3 by the JAK/STAT pathway, could result in the inhibition of insulin signaling. Hence, obese mice lacking LRAT or STRA6 appear to be protected from retinol/STRA6-induced insulin resistance (17, 18).
Regulatory capacity of retinal
Apart from its role as a ligand for opsin in the visual cascade (see Visual system and eyesight), retinal has been specifically implicated in the regulation of genes important for lipid metabolism. In humans, two types of adipose tissue have been distinguished based on their respective functions: white adipose tissue (WAT) stores fatty acids as triglycerides, and brown adipose tissue (BAT) oxidizes fatty acids to generate heat (thermogenesis). In the mitochondrial respiratory chain of brown adipose cells, the processes of electron transport and ATP production are uncoupled (dissociated) to permit the rapid production of heat from fatty acid oxidation (19).
Retinaldehyde dehydrogenase 1 (RALDH1), which converts retinal to retinoic acid, is highly expressed in WAT but not in BAT. The suppression of RALDH1 expression in WAT can induce a thermogenic phenotype resembling that of BAT (20). During adipocyte differentiation, the stimulation of cells with all-trans retinal has been found to activate the UCP1 gene required for thermogenesis while inhibiting genes promoting adipogenesis, such as PPARγ (20). Retinal also appeared to regulate lipid metabolism and adiposity in bone marrow by inhibiting PPARγ/RXR heterodimer-mediated gene expression (21). In addition, retinal was found to inhibit gluconeogenic gene expression and glucose production in the liver of mice deficient in RALDH1 (22).
Immunity
Vitamin A was initially coined "the anti-infective vitamin" because of its importance in the normal functioning of the immune system (23). The skin and mucosal cells, lining the airways, digestive tract, and urinary tract function as a barrier and form the body's first line of defense against infection. Retinoic acid (RA) is produced by antigen-presenting cells (APCs), including macrophages and dendritic cells, found in these mucosal interfaces and associated lymph nodes. RA appears to act on dendritic cells themselves to regulate their differentiation, migration, and antigen-presenting capacity. In addition, the production of RA by APCs is required for the differentiation of naïve CD4 T-lymphocytes into induced regulatory T- lymphocytes (Tregs). Critical to the maintenance of mucosal integrity, the differentiation of Tregs is driven by all-trans-RA through RARα-mediated regulation of gene expression (see Regulation of gene expression). Also, during inflammation, all-trans-RA/RARα signaling pathway promotes the conversion of naïve CD4 T-lymphocytes into effector T-lymphocytes − type 1 helper T-cells (Th1) − (rather than into Tregs) and induces the production of proinflammatory cytokines by effector T-lymphocytes in response to infection. There is also substantial evidence to suggest that RA may help prevent the development of autoimmunity (reviewed in 24).
Prenatal and postnatal development
Both vitamin A excess and deficiency are known to cause birth defects. Retinoid signaling begins soon after the early phase of embryonic development known as gastrulation. During fetal development, RA is critical for the development of organs, including the heart, eyes, ears, lungs, as well as other limbs and visceral organs. Vitamin A has been implicated in fetal lung maturation (2). Vitamin A status is lower in preterm newborns than in full-term infants (25). There is some evidence to suggest that vitamin A supplementation may help reduce the incidence of chronic lung disease and mortality in preterm newborns (see Disease Prevention). Retinoid signaling is also involved in the expression of many proteins of the extracellular matrix (ECM; material surrounding cells), including collagen, laminin, and proteoglycans (26). Vitamin A deficiency may then result in alterations of the ECM composition, thus disrupting organ morphology and function (reviewed in 26).
Red blood cell production(erythropoiesis)
Red blood cells (erythrocytes), like all blood cells, are derived from pluripotent stem cells in the bone marrow. Studies involving in vitro culture systems have suggested a role for retinoids in stem cell commitment and differentiation to the red blood cell lineage. Retinoids might also regulate apoptosis (programmed cell death) of red blood cell precursors (erythropoietic progenitor cells) (27). However, whether retinoids regulate erythropoiesis in vivo has not been established. Yet, vitamin A supplementation in vitamin A deficient-individuals has been shown to increase hemoglobin concentrations. Additionally, vitamin A appears to facilitate the mobilization of iron from storage sites to the developing red blood cell for incorporation into hemoglobin, the oxygen carrier in red blood cells (27, 28).
Nutrient interactions
Zinc
Zinc deficiency is thought to interfere with vitamin A metabolism in several ways (29): (1) zinc deficiency results in decreased synthesis of retinol-binding protein (RBP), which transports retinol through the circulation to peripheral tissues and protects the organism against potential toxicity of retinol; (2) zinc deficiency results in decreased activity of the enzyme that releases retinol from its storage form, retinyl palmitate, in the liver; and (3) zinc is required for the enzyme that converts retinol into retinal (30). The health consequences of zinc deficiency on vitamin A nutritional status in humans are yet to be defined (29).
Iron
Vitamin A deficiency often coexists with iron deficiency and may exacerbate iron deficiency anemia by altering iron metabolism (27). Vitamin A supplementation has beneficial effects on iron deficiency anemia and improves iron nutritional status among children and pregnant women (27, 28). The combination of supplemental vitamin A and iron seems to reduce anemia more effectively than either supplemental iron or vitamin A alone (31). Moreover, studies in rats have shown that iron deficiency alters plasma and liver levels of vitamin A (32, 33).
Deficiency
Vitamin A deficiency usually results from inadequate intakes of vitamin A from animal products (as preformed vitamin A) and fruit and vegetables (as provitamin A carotenoids). In developing countries, vitamin A deficiency and associated disorders predominantly affect children and women of reproductive age. Other individuals at risk of vitamin A deficiency are those with poor absorption of lipids due to impaired pancreatic or biliary secretion and those with inflammatory bowel diseases, such as Crohn's disease and celiac disease (2). Subclinical vitamin A deficiency is often defined by serum retinol concentrations lower than 0.70 μmol/L (20 μg/dL). In severe vitamin A deficiency, vitamin A body stores are depleted and serum retinol concentrations fall below 0.35 μmol/L (10 μg/dL). Other biomarkers have been calibrated to assess vitamin A nutritional status (reviewed in 34). Of note, the World Health Organization considers vitamin A deficiency a public health problem when the prevalence of low serum retinol (<0.70 μmol/L) reaches 15% or more of a defined population.
Vitamin A deficiency-related disorders
Disease of the eye and blindness
With an estimated 250,000 to 500,000 children becoming blind annually, vitamin A deficiency constitutes the leading preventable cause of blindness in low- and middle-income nations (35). The earliest symptom of vitamin A deficiency is impaired dark adaptation known as night blindness or nyctalopia. The next clinical stage is the occurrence of abnormal changes in the conjunctiva (corner of the eye), manifested by the presence of Bitot's spots. Severe or prolonged vitamin A deficiency eventually results in a condition called xerophthalmia (Greek for dry eye), characterized by changes in the cells of the cornea (clear covering of the eye) that ultimately result in corneal ulcers, scarring, and blindness (36). Immediate administration of 200,000 international units (IU) of vitamin A for two consecutive days is required to prevent blinding xerophthalmia (36).
There is an estimated 19.1 million pregnant women worldwide (especially in Sub-Saharan Africa, Southeast Asia, and Central America) with vitamin A deficiency and over half of them are affected by night blindness (37). The prevalence of vitamin A deficiency and night blindness is especially high during the third trimester of pregnancy due to accelerated fetal growth. Also, approximately 190 million preschool-age children have low serum retinol concentrations (<0.70 μmol/L), with 5.2 million suffering from night blindness. Moreover, half of the children affected by severe vitamin A deficiency-induced blinding xerophthalmia are estimated to die within a year of becoming blind (37). The World Health Organization (WHO) and the United Nations Children's Fund (UNICEF) promote vitamin A supplementation as a public health intervention to reduce child mortality in areas and populations where vitamin A deficiency is prevalent (38-40).
Susceptibility to infectious diseases
Infectious diseases have been associated with depletion of vitamin A hepatic reserves (already limited in vitamin A-deficient subjects), reduced serum retinol concentrations, and increased loss of vitamin A in the urine (37). Infection with the measles virus was found to precipitate conjunctival and corneal damage, leading to blindness in children with poor vitamin A status (41). Conversely, vitamin A deficiency can be considered a nutritionally acquired immunodeficiency disease (42). Even children who are only mildly deficient in vitamin A have a higher incidence of respiratory complications and diarrhea, as well as a higher rate of mortality from measles infection compared to children consuming sufficient vitamin A (43). Because vitamin A supplementation may decrease both the severity and incidence of measles complications in developing countries (see Disease Prevention), WHO recommends that children aged at least one year receive 200,000 IU of vitamin A (60 mg RAE) for two consecutive days in addition to standard treatment when they are infected with measles virus and live in areas of vitamin A deficiency (44).
A recent prospective cohort study, conducted in 2,774 Colombian children (ages, 5-12 years old) followed for a median 128 days, also reported an inverse relationship between plasma retinol concentrations and rates of diarrhea with vomiting and cough with fever, the latter being a strong predictor of influenza infection (flu) (45). A review of five randomized, placebo-controlled studies that included 7,528 HIV-positive pregnant or breast-feeding women found no substantial benefit of vitamin A supplementation in reducing the mother-to-child transmission of HIV (46). One early observational study found that HIV-infected women who were vitamin A deficient were three to four times more likely to transmit HIV to their infants (47). Yet, no trial to date has provided any information on potential adverse effects of vitamin A supplementation on mother-to-child HIV transmission (48).
Thyroid dysfunction
In North and West Africa, vitamin A deficiency and iodine deficiency induced-goiter can coexist in up to 50% of children. The response to iodine prophylaxis in iodine-deficient populations appears to depend on various nutritional factors, including vitamin A status (49, 50). Vitamin A deficiency in animal models was found to interfere with the pituitary-thyroid axis by (1) increasing the synthesis and secretion of thyroid-stimulating hormone (TSH) by the pituitary gland, (2) increasing the size of the thyroid gland, (3) reducing iodine uptake by the thyroid gland and impairing the synthesis and iodination of thyroglobulin, and (4) increasing circulating concentrations of thyroid hormones (reviewed in 51). A cross-sectional study of 138 children with concurrent vitamin A and iodine deficiencies found that the severity of vitamin A deficiency was associated with higher risk of goiter and higher concentrations of circulating TSH and thyroid hormones (50). These children received iodine-enriched salt with either vitamin A (200,000 IU at baseline and 5 months) or placebo in a randomized, double-blind, 10-month trial. This vitamin A supplementation significantly decreased TSH concentration and thyroid volume compared to placebo (50). In another trial, supplementation of vitamin A to iodine-deficient children had no additional effect to iodine on thyroid status compared to placebo, but vitamin A supplementation alone (without iodine) reduced the volume of the thyroid gland, as well as TSH and thyroglobulin concentrations (52).
Other disorders
Phrynoderma or follicular hyperkeratosis is a skin condition characterized by an excessive production of keratin in hair follicles. The lesions first appear on the extremities, shoulders, and buttocks and may spread over the entire body in the severest cases (53). While vitamin A deficiency may contribute to the occurrence of phrynoderma, the condition has been strongly associated with multiple nutritional deficiencies and is considered a sign of general malnutrition. A rare case of esophagitis (inflammation of the esophagus) has recently been attributed to hyperkeratosis secondary to vitamin A deficiency (54).
Also, vitamin A deficiency affects iron mobilization, impairs hemoglobin synthesis, and precipitates iron deficiency anemia that is only alleviated with supplementation of both vitamin A and iron (see Nutrient interactions) (27).
The Recommended Dietary Allowance (RDA)
Retinol Activity Equivalents (RAE)
Vitamin A can be obtained from food as preformed vitamin A in animal products or as provitamin A carotenoids in fruit and vegetables (see Food sources). Yet, while preformed vitamin A is effectively absorbed, stored, and hydrolyzed to form retinol, provitamin A carotenoids like β-carotene are less easily digested and absorbed, and must be converted to retinol and other retinoids by the body after uptake into the small intestine. The efficiency of conversion of provitamin A carotenes into retinol is highly variable, depending on factors such as food matrix, food preparation, and one's digestive and absorptive capacities (55).
The most recent international standard of measure for vitamin A is retinol activity equivalents (RAE), which represent vitamin A activity as retinol. It has been determined that 2 micrograms (μg) of β-carotene in oil provided as a supplement could be converted by the body to 1 μg of retinol giving it an RAE ratio of 2:1. However, 12 μg of β-carotene from food are required to provide the body with 1 μg of retinol, giving dietary β-carotene an RAE ratio of 12:1. Other provitamin A carotenoids in food are less easily absorbed than β-carotene, resulting in RAE ratios of 24:1. RAE ratios are shown in Table 1 (56).
| Quantity Consumed | Quantity Bioconverted to Retinol | RAE Ratio |
|---|---|---|
| 1 μg of dietary or supplemental vitamin A | 1 μg of retinol* | 1:1 |
| 2 μg of supplemental β-carotene | 1 μg of retinol | 2:1 |
| 12 μg of dietary β-carotene | 1 μg of retinol | 12:1 |
| 24 μg of dietary α-carotene | 1 μg of retinol | 24:1 |
| 24 μg of dietary β-cryptoxanthin | 1 μg of retinol | 24:1 |
| *1 IU is equivalent to 0.3 microgram (μg) of retinol, and 1 μg of retinol is equivalent to 3.33 IU of retinol. | ||
Determination of the RDA
The RDA for vitamin A was revised by the Food and Nutrition Board (FNB) of the US Institute of Medicine (IOM) in 2001. The RDA is based on the Estimated Average Requirement (EAR), which is defined as the biological requirement for 50% of the population. The RDA is the recommended intake needed by nearly all of the population to ensure adequate hepatic stores of vitamin A in the body (20 μg/g for four months if the person consumes a vitamin A-deficient diet) to support normal reproductive function, immune function, gene expression, and vision (for details of calculations, see 56). Table 2 lists the RDA values in micrograms (μg) of Retinol Activity Equivalents (RAE) per day.
| Life Stage | Age | Males (μg/day) | Females (μg/day) |
|---|---|---|---|
| Infants | 0-6 months | 400 (AI) | 400 (AI) |
| Infants | 7-12 months | 500 (AI) | 500 (AI) |
| Children | 1-3 years | 300 | 300 |
| Children | 4-8 years | 400 | 400 |
| Children | 9-13 years | 600 | 600 |
| Adolescents | 14-18 years | 900 | 700 |
| Adults | 19 years and older | 900 | 700 |
| Pregnancy | 18 years and younger | - | 750 |
| Pregnancy | 19 years and older | - | 770 |
| Breast-feeding | 18 years and younger | - | 1,200 |
| Breast-feeding | 19 years and older | - | 1,300 |
Disease Prevention
Bronchopulmonary dysplasia in preterm infants
Preterm infants are born with inadequate body stores of vitamin A, placing them at risk of developing diseases of the eye and the respiratory and gastrointestinal tracts. About one-third of preterm infants born between 22 and 28 weeks of gestation develop bronchopulmonary dysplasia (BPD), a chronic lung disease that can be fatal or result in life-long morbidities in survivors. A few randomized controlled studies have investigated the effect of postnatal vitamin A supplementation on the incidence of BPD and the risk of mortality in very low birth weight infants (≤1,500 g) requiring respiratory support (57-59). In the largest, multicenter, randomized, blinded, placebo-controlled trial that enrolled 807 extremely low birth weight (ELBW; ≤1,000 g) preterm newborns, the intramuscular administration of 5,000 IU of vitamin A three times a week for four weeks significantly, though modestly, reduced the risk of BPD or death at 36 weeks' postmenstrual age (gestational age plus chronological age) (58). While vitamin A supplementation was included in some neonatal programs after this trial (60), a national shortage in vitamin A supply that has affected US neonatal intensive care units since 2010 has led to a significant reduction in the use of vitamin A supplementation in premature newborns (401-1,000 g at birth) with respiratory failure (61, 62). However, a retrospective analysis of US nationwide data from 6,210 preterm infants born between 2010 and 2012 found that a reduction in vitamin A prophylaxis from 27.2% to 2.1% during the same period had no significant impact on the incidence of BPD or death before hospital discharge (62).
In another retrospective study, the nonrandomized use of vitamin A supplementation with inhaled nitric oxide (iNO) was found to result in a lower incidence of BPD (but not mortality) compared to iNO therapy alone in preterm newborns with a birth weight of 750-999 g (63). Neurodevelopment index scores at one year of age were also improved in the vitamin A group of newborns weighing 500-749 g at birth. Yet, caution is advised with the interpretation of the results, especially because the trial was not designed to assess the effect of vitamin A. In Germany, one large, multicenter, randomized study – the NeoVitaA trial – is under way to explore the effect of high-dose oral vitamin A (5,000 IU/kg/day) for 28 days on the incidence of BPD and mortality at 36 weeks' postmenstrual age (64).
While high doses of vitamin A during early pregnancy can cause birth defects (see Safety), vitamin A supplementation during late pregnancy may improve maternal and fetal vitamin A status (65). Although a few randomized controlled trials have failed to show an effect on maternal and neonatal mortality (66), more research is required to assess whether vitamin A supplementation during pregnancy reduces BPD incidence in infants.
Childhood morbidity and mortality
A recent meta-analysis of randomized controlled trials evaluating the preventive effect of vitamin A on childhood mortality indicated that vitamin A supplementation (200,000 IU every 4 or 6 months) reduced all-cause mortality by 25% (13 studies) and diarrhea-specific mortality by 30% (7 studies) in children aged 6 to 59 months. However, vitamin A administration in this age group had no preventive effect on rates of pneumonia-specific mortality (7 studies), measles-specific mortality (5 studies), or meningitis-specific mortality (3 studies). Moreover, no reduction in the risk of disease-specific mortality was found in neonates (0 to 28 days of age) and infants 1 to 6 months of age supplemented with vitamin A (67). Another meta-analysis of randomized controlled trials found no evidence of a reduction in mortality risk during infancy when either breast-feeding mothers (7 studies) or infants aged less than six months (9 studies) were supplemented with vitamin A (68).
Current WHO policy recommends vitamin A supplementation at routine vaccination contacts in children after six months of age living in regions at high risk of vitamin A deficiency. Supplementation with high doses of vitamin A − 100,000 IU (30 mg RAE) for infants 6 to 11 months of age and 200,000 IU (60 mg RAE) for children 12 to 59 months of age − is thought to provide adequate protection for up to six months (38). A recent placebo-controlled trial in Guinea-Bissau, which randomized 7,587 children (ages, 6 to 23 months old) to receive vitamin A supplementation at one vaccination contact, evaluated the co-administration of vitamin A and vaccines on child mortality (69). The study found that vitamin A supplementation had no effect on overall mortality rates, although a six-month follow-up of infants given both measles and DTP (diphtheria-tetanus-pertussis) vaccinations showed a significant reduction in mortality in girls, but not in boys (69). Although neonatal vitamin A supplementation is not currently recommended, a trial assessing the benefit of early measles vaccination − at 4.5 rather than the usual 9 months of age − found no reduction in mortality rates when children had received neonatal vitamin A supplementation (70). The recent pooled analysis of previous trials of vitamin A supplementation (VITA I-III) in Guinea-Bissau confirmed that vitamin A supplementation may interfere with vaccines. Specifically, compared to placebo, neonatal vitamin A supplementation was associated with a significant increase in mortality rates in boys (but not in girls) when children had received measles virus vaccination at 4.5 months of age rather than the usual 9 months of age (71). The timing of vitamin A interventions needs to be further examined in relation to the timing of vaccinations in order to maximize their benefits.
Complications from measles infection
An earlier meta-analysis of seven randomized controlled trials examining specifically the role of vitamin A supplementation in 2,069 children with measles found no overall reduction on the risk of mortality (72). Yet, the pooled analysis of four studies that reported the age distribution of participants found an 83% lower risk of mortality with two doses of 200,000 IU of vitamin A in children younger than two years. In addition, the pooled analysis of three studies indicated a 67% reduction in the risk of pneumonia-led mortality (72). Similar to WHO and UNICEF guidelines, the American Academy of Pediatrics recommends vitamin A supplementation for children over six months of age when they are infected with measles while malnourished, immunodeficient, or are at risk of measles complications or vitamin A deficiency disorders (73). Although measles infection has been associated with vitamin A deficiency and blindness, there is currently no evidence to suggest that vitamin A supplementation reduces the risk of blindness in children infected with measles (74).
Cancer
Studies in cell culture and animal models have documented the capacity for natural and synthetic retinoids to reduce carcinogenesis significantly in skin, breast, liver, colon, prostate, and other sites. However, the results of human studies examining the relationship between the consumption of preformed vitamin A and cancer do not currently suggest that consuming vitamin A at intakes greater than the RDA benefit in the prevention of cancer (2).
Lung cancer
The results of the β-Carotene And Retinol Efficacy Trial (CARET) have suggested that high-dose supplementation of preformed vitamin A and β-carotene should be avoided in people at high risk for lung cancer (75). In the CARET study, about 9,000 people (smokers and people with asbestos exposure) were assigned a daily regimen of 25,000 IU (7,500 μg RAE) of retinyl palmitate and 30 mg of β-carotene, while a similar number of people were assigned a placebo. After four years of follow-up, the incidence of lung cancer was 28% higher in the supplemented group compared to the placebo group; however, the incidence was not different six years after the intervention ended (76). A possible explanation for an increase in lung cancer is that the oxidative environment of the lung, created by smoke or asbestos exposure, could give rise to unusual carotenoid cleavage products, which might promote carcinogenesis (77). Interestingly, a case-control study that included 749 lung cancer cases and 679 controls from the CARET trial found a significant association between lung cancer risk reduction and high vitamin D intakes (≥400 IU/day) in individuals who received the active CARET supplements or in those with vitamin A intakes equal to or greater than 1,500 μg RAE/day (78). Further, a recent meta-analysis of four randomized controlled trials, including a total of 202,924 participants at low risk of lung cancer, indicated that supplementation with retinol and/or β-carotene had no significant effect on lung cancer incidence (79). At present, it seems unlikely that increased intake of preformed vitamin A (e.g., retinol) could lower the risk of lung cancer.
Disease Treatment
Retinoids may be used at pharmacological doses to treat several conditions, including, acute promyelocytic leukemia, retinitis pigmentosa, and various skin diseases. It is important to note that treatment with high doses of natural or synthetic retinoids overrides the body's own control mechanisms; therefore, retinoid therapies are associated with potential side effects and toxicities. Additionally, all of the retinoid compounds have been found to cause fetal deformations. Thus, women who have a chance of becoming pregnant should avoid treatment with these medications. Retinoids tend to be very long acting: side effects and birth defects have been reported to occur months after discontinuing retinoid therapy (2). The retinoids discussed below are prescription drugs and should not be used without medical supervision.
Acute promyelocytic leukemia
Normal differentiation of myeloid stem cells in the bone marrow gives rise to platelets, red blood cells, and white blood cells (also called leukocytes) that are important for the immune response. Altered differentiation of myeloid cells can result in the proliferation of immature white blood cells, giving rise to leukemia. Reciprocal chromosome translocations involving the promyelocytic leukemia (PML) gene and the gene coding for retinoic acid receptor α (RARα) lead to a specific type of leukemia called acute promyelocytic leukemia (APL). The fusion protein PML/RARα represses transcription by binding to RARE in the promoter of retinoid-responsive genes involved in hematopoietic cell differentiation. Gene repression by PML/RARα is achieved by the recruitment of several chromatin modifiers, including histone deacetylases (HDACs) and DNA methyltransferases (DNMTs). Contrary to RARα wild-type receptor, PML/RARα appears to be insensitive to physiological concentrations of retinoic acid (RA) such that only treatments with high doses of all-trans-RA can restore normal differentiation and lead to significant improvements and complete remission in some APL patients (80).
More information on APL treatment programs can be found in the National Cancer Institute website.
Diseases of the skin
Both natural and synthetic retinoids have been used as pharmacologic agents to treat disorders of the skin. Acitretin is a synthetic retinoid that has been proven useful in combination treatments for psoriasis (81). Topical tretinoin (all-trans-retinoic acid) and oral isotretinoin (13-cis-retinoic acid) have been used successfully to treat mild-to-severe acne vulgaris (82, 83). Retinoids exhibit anti-inflammatory properties and regulate the proliferation and differentiation of skin epithelial cells, as well as the production of sebum. Use of pharmacological doses of retinoids (especially oral isotretinoin) by pregnant women causes birth defects and is therefore contraindicated prior to and during pregnancy (see Safety in pregnancy).
For more information on the use of retinoids in the management of acne, see the article on Vitamin A and Skin Health.
Retinitis pigmentosa
Retinitis pigmentosa (RP) affects approximately 1.5 million people worldwide and is a leading cause of inherited blindness. RP describes a broad spectrum of genetic disorders that result in the progressive loss of photoreceptor cells (rods and cones) in the retina of the eye (84). While at least 45 loci have been associated with RP, mutations in the rhodopsin gene (RHO), the usherin gene (USH2A), and the RP GTPase regulator gene (RPGR) account for about 30% of all RP cases (85).
Early symptoms of RP include impaired dark adaptation and night blindness, followed by the progressive loss of peripheral and central vision over time (85). The results of only one randomized controlled trial in 601 patients with common forms of RP indicated that supplementation with 15,000 IU/day of retinyl palmitate (4,500 μg RAE) significantly slowed the loss of retinal function over a period of four to six years (86). In contrast, supplementation with 400 IU/day of vitamin E (dl-α-tocopherol) modestly but significantly increased the loss of retinal function, suggesting that patients with common forms of RP may benefit from long-term vitamin A supplementation but should avoid high-dose vitamin E supplementation. Up to 12 years of follow-up in these patients did not reveal any signs of liver toxicity as a result of excess vitamin A intake (87). Because neither children younger than 18 years nor adults affected by less common forms of RP were included in the trial, no formal recommendation about vitamins A and E could be made (85). High-dose vitamin A supplementation to slow the course of RP requires medical supervision and must be discontinued if there is a possibility of pregnancy (see Safety).
Sources
Food sources
Free retinol is not generally found in food. Retinyl esters (including retinyl palmitate) are the storage form of retinol in animals and thus the main precursors of retinol in food from animals. Plants contain carotenoids, some of which are precursors for vitamin A (e.g., α-carotene, β-carotene, and β-cryptoxanthin). Yellow- and orange-colored vegetables contain significant quantities of carotenoids. Green vegetables also contain carotenoids, though yellow-to-red pigments are masked by the green pigment of chlorophyll (1). The table below lists a number of good food sources of vitamin A, including fruit and vegetables, along with their vitamin A content. The retinol activity is indicated in micrograms of retinol activity equivalents (μg RAE). For information on this unit of measurement, see the section on RAE. In addition, use USDA's FoodData Central to check foods for their content of carotenoids without vitamin A activity, such as lycopene, lutein, and zeaxanthin.
Vitamin A international units (IUs)
Vitamin A may still be listed on food and supplement labels in international units versus as μg RAE ('mcg' on labels). USDA's FoodData Central also provides the vitamin A content of food sources using the vitamin A international unit (IU). Yet, contrary to RAE, the number of IUs of vitamin A does not reflect the bioavailability of vitamin A from different food sources. Conversion rates between IUs and μg RAE are set as follows:
• 1 IU of retinol is equivalent to 0.3 μg RAE
• 1 IU of supplemental β-carotene is equivalent to 0.3 μg RAE
• 1 IU of dietary β-carotene is equivalent to 0.05 μg RAE
• 1 IU of α-carotene or β-cryptoxanthin to 0.025 μg RAE
Thus, in Table 3, the number of IUs of vitamin A in carotenoid-containing food (numbers in italics) can be obtained by multiplying the RAE by approximately 20.
| Food | Serving | Preformed Vitamin A (Retinol), μg | Vitamin A, μg RAE | Vitamin A, IU |
|---|---|---|---|---|
| Beef liver, cooked | 1 slice (68 g) | 6,421* | 6,421* | 21,566* |
| Cod liver oil | 1 teaspoon | 1,350 | 1,350 | 4,500 |
| Fortified breakfast cereal (oats) | 1 serving (1 oz) | 216 | 216 | 721 |
| Egg | 1 large | 80 | 80 | 270 |
| Butter | 1 tablespoon | 95 | 95 | 355 |
| Whole milk | 1 cup (8 fl oz) | 110 | 110 | 395 |
| 2% fat milk (vitamin A added) | 1 cup (8 fl oz) | 134 | 134 | 464 |
| Nonfat milk (vitamin A added) | 1 cup (8 fl oz) | 149 | 149 | 500 |
| Sweet potato (canned, mashed) | ½ cup | 0 | 555 | 11,091 |
| Sweet potato (baked) | ½ cup | 0 | 961 | 19,218 |
| Pumpkin (canned) | ½ cup | 0 | 953 | 19,065 |
| Carrot (raw, chopped) | ½ cup | 0 | 534 | 10,692 |
| Cantaloupe | ½ medium melon | 0 | 466 | 9,334 |
| Mango | 1 fruit | 0 | 181 | 3,636 |
| Spinach (cooked) | ½ cup | 0 | 472 | 9,433 |
| Broccoli (cooked) | ½ cup | 0 | 60 | 1,207 |
| Kale (cooked) | ½ cup | 0 | 443 | 8,854 |
| Collards (cooked) | ½ cup | 0 | 361 | 7,220 |
| Squash, butternut (cooked) | ½ cup | 0 | 572 | 11,434 |
| *Above the tolerable upper intake level (UL) of 3,000 μg RAE (10,000 IU)/day | ||||
Supplements
The principal forms of preformed vitamin A in supplements are retinyl palmitate and retinyl acetate. β-Carotene is also a common source of vitamin A in supplements, and many supplements provide a combination of retinol and β-carotene (88). If a percentage of the total vitamin A content of a supplement comes from β-carotene, this information is included in the Supplement Facts label under vitamin A (Figure 4). Some multivitamin supplements available in the US provide up to 5,000 IU of preformed vitamin A, corresponding to 1,500 μg RAE, which is substantially more than the current RDA for vitamin A. This is due to the fact that the Daily Values (DV) used by the US Food and Drug Administration (FDA) for supplement labeling are based on the RDA established in 1968 rather than the most recent RDA, and multivitamin supplements typically provide 100% of the DV for most nutrients. Because retinol intakes of 5,000 IU/day (1,500 μg RAE) may be associated with an increased risk of osteoporosis in older adults (see Safety), some companies have reduced the retinol content in their multivitamin supplements to 2,500 IU (750 μg RAE).

Safety
Toxicity
The condition caused by vitamin A toxicity is called hypervitaminosis A. It is caused by overconsumption of preformed vitamin A, not carotenoids. Preformed vitamin A is rapidly absorbed and slowly cleared from the body. Therefore, toxicity from preformed vitamin A may result acutely from high-dose exposure over a short period of time or chronically from a much lower intake (2). Acute vitamin A toxicity is relatively rare, and symptoms include nausea, headache, fatigue, loss of appetite, dizziness, dry skin, desquamation, and cerebral edema. Signs of chronic toxicity include dry itchy skin, desquamation, anorexia, weight loss, headache, cerebral edema, enlarged liver, enlarged spleen, anemia, and bone and joint pain. Also, symptoms of vitamin A toxicity in infants include bulging fontanels. Severe cases of hypervitaminosis A may result in liver damage, hemorrhage, and coma. Generally, signs of toxicity are associated with long-term consumption of vitamin A in excess of 10 times the RDA (8,000-10,000 μg RAE/day or 25,000-33,000 IU/day). However, more research is necessary to determine if subclinical vitamin A toxicity is a concern in certain populations (89). There is evidence that some populations may be more susceptible to toxicity at lower doses, including the elderly, chronic alcohol users, and some people with a genetic predisposition to high cholesterol (90). In January 2001, the Food and Nutrition Board of the US Institute of Medicine set the tolerable upper intake level (UL) of vitamin A intake for adults at 3,000 μg RAE (10,000 IU)/day of preformed vitamin A (56).
| Age Group | μg RAE/day | IU/day* |
|---|---|---|
| Infants 0-12 months | 600 | 2,000 |
| Children 1-3 years | 600 | 2,000 |
| Children 4-8 years | 900 | 3,000 |
| Children 9-13 years | 1,700 | 5,667 |
| Adolescents 14-18 years | 2,800 | 9,333 |
| Adults 19 years and older | 3,000 | 10,000 |
| *1 IU of preformed vitamin A is equivalent to 0.3 μg RAE, and 1 μg RAE is equivalent to 3.33 IU of preformed vitamin A | ||
Safety in pregnancy
Although normal fetal development requires sufficient vitamin A intake, consumption of excess preformed vitamin A (such as retinol) during early pregnancy is known to cause birth defects. No increase in the risk of vitamin A-associated birth defects has been observed at doses of preformed vitamin A from supplements below 3,000 μg RAE/day (10,000 IU/day) (56). Of note, in 2011, the World Health Organization (WHO) recommended vitamin A supplementation (up to 3,000 μg RAE/day or 7,500 μg RAE/week) during pregnancy in areas with high prevalence of vitamin A deficiency for the prevention of blindness (91). In industrialized countries, pregnant or potentially pregnant women should monitor their intake of vitamin A from fortified food and food naturally high in preformed vitamin A (e.g., liver) and avoid taking daily multivitamin supplements that contain more than 1,500 μg RAE (5,000 IU) of vitamin A. There is no evidence that consumption of vitamin A from β-carotene might increase the risk of birth defects. The synthetic derivative of retinol, isotretinoin, is known to cause serious birth defects and should not be taken during pregnancy or if there is a possibility of becoming pregnant (82). Tretinoin (all-trans-retinoic acid), another retinol derivative, is prescribed as a topical preparation that is applied to the skin. Although percutaneous absorption of topical tretinoin is minimal, its use during pregnancy is not recommended (92).
Do high intakes of vitamin A increase the risk of osteoporosis?
Results from some prospective studies have suggested that long-term intakes of preformed vitamin A in excess of 1,500 μg RAE/day (equivalent to 5,000 IU/day of vitamin A as retinol) were associated with reduced bone mineral density (BMD) and increased risk of osteoporotic fracture in older adults (93-95). However, other investigators failed to observe such detrimental effects on BMD and/or fracture risk (96-98). The recent meta-analysis of four prospective studies, including nearly 183,000 participants over 40 years of age, found that highest vs. lowest quintiles of retinol (preformed vitamin A) intake significantly increased the risk of hip fracture (99). Only excess intakes of retinol, not β-carotene, were associated with adverse effects on bone health. Besides, the pooled analysis of four observational studies also indicated that a U-shaped relationship between circulating retinol and risk of hip fracture, suggesting that both elevated and reduced retinol concentrations in the blood were associated with an increased risk of hip fracture (99).
To date, limited experimental data have suggested that vitamin A (as all-trans-retinoic acid) may affect the development of bone-remodeling cells and stimulate bone matrix degradation (resorption) (reviewed in 100). Vitamin A may also interfere with the ability of vitamin D to maintain calcium balance (101). In the large Women's Health Initiative (WHI) prospective study, the highest vs. lowest quintile of retinol intake (≥1,426 μg/day vs. <474 μg/day) was found to be significantly associated with increased risk of fracture only in women with the lowest vitamin D intakes (≤440 IU/day) (102).
Until supplements and fortified food are reformulated to reflect the current RDA for vitamin A, it is advisable for older individuals to consume multivitamin supplements that contain no more than 2,500 IU (750 μg) of preformed vitamin A (usually labeled vitamin A acetate or vitamin A palmitate) and no more than 2,500 IU of additional vitamin A as β-carotene.
Drug interactions
Chronic alcohol consumption results in depletion of liver stores of vitamin A and may contribute to alcohol-induced liver damage (cirrhosis) (103). However, the liver toxicity of preformed vitamin A (retinol) is enhanced by chronic alcohol consumption, thus narrowing the therapeutic window for vitamin A supplementation in alcoholics (103). Oral contraceptives that contain estrogen and progestin increase retinol binding protein (RBP) synthesis by the liver, increasing the export of all-trans-retinol/RBP complex to the circulation. Whether this increases the dietary requirement of vitamin A is not known. Also, the use of cholesterol-lowering medications (like cholestyramine and colestipol), as well as orlistat, mineral oil, and the fat substitute, olestra, which interfere with fat absorption, may affect the absorption of fat-soluble vitamins, including vitamin A (88). Further, intake of large doses of vitamin A may decrease the absorption of vitamin K. Retinoids or retinoid analogs, including acitretin, all-trans-retinoic acid, bexarotene, etretinate, and isotretinoin, should not be used in combination with single-nutrient vitamin A supplements, because they may increase the risk of vitamin A toxicity (88).
Linus Pauling Institute Recommendation
The RDA for vitamin A (700 μg RAE/day for women and 900 μg RAE/day for men) is sufficient to support normal gene expression, immune function, and vision. However, following the Linus Pauling Institute's recommendation to take a multivitamin/mineral supplement daily could supply as much as 5,000 IU (1,500 μg RAE)/day of vitamin A as retinol, the amount that has been associated with adverse effects on bone health in older adults. For this reason, we recommend taking a multivitamin/mineral supplement that provides no more than 2,500 IU (750 μg) of preformed vitamin A (usually labeled vitamin A acetate or vitamin A palmitate) and no more than 2,500 IU of additional vitamin A as β-carotene. High potency vitamin A supplements should not be used without medical supervision due to the risk of toxicity.
Older adults (>50 years)
Presently, there is little evidence that the requirement for vitamin A in older adults differs from that of younger adults. Additionally, vitamin A toxicity may occur at lower doses in older adults than in younger adults. Further, data from observational studies suggested an association between intakes of preformed vitamin A in excess of 1,500 μg RAE (5,000 IU)/day and increased risk of hip fracture in older people (see Safety). Yet, following the Linus Pauling Institute's recommendation to take a multivitamin/mineral supplement daily could supply as much as 5,000 IU/day of retinol, the amount that has been associated with adverse effects on bone health in older adults. For this reason, we recommend taking a multivitamin/mineral supplement that provides (750 μg) of preformed vitamin A (usually labeled vitamin A acetate or vitamin A palmitate) and no more than 2,500 IU of additional vitamin A as β-carotene. As for all age groups, high potency vitamin A supplements should not be used without medical supervision due to the risk of toxicity.
Authors and Reviewers
Originally written in 2000 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in December 2003 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in November 2007 by:
Victoria J. Drake, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in January 2015:
Barbara Delage, Ph.D.
Linus Pauling Institute
Oregon State University
Reviewed in February 2015 by:
A. Catharine Ross, Ph.D.
Professor of Nutrition
Dorothy Foehr Huck Chair
Department of Nutritional Sciences
The Pennsylvania State University
Reviewed in March 2015 by:
Libo Tan, Ph.D.
Assistant Professor
Department of Human Nutrition
The University of Alabama
Last updated 2/25/21 Copyright 2000-2021 Linus Pauling Institute
References
1. Groff JL. Advanced Nutrition and Human Metabolism. 2nd ed. St. Paul: West Publishing; 1995.
2. Ross AC. Vitamin A. In: Ross A, Caballero B, Cousins R, Tucker K, Ziegler T, eds. Modern Nutrition in Health and Disease. 11th ed: Lippincott Williams & Wilkins; 2014:260-277.
3. Tan L, Green MH, Ross AC. Vitamin A Kinetics in Neonatal Rats vs. Adult Rats: Comparisons from Model-Based Compartmental Analysis. J Nutr. 2014;145(3):403-410. (PubMed)
4. Tan L, Wray AE, Green MH, Ross AC. Compartmental modeling of whole-body vitamin A kinetics in unsupplemented and vitamin A-retinoic acid-supplemented neonatal rats. J Lipid Res. 2014;55(8):1738-1749. (PubMed)
5. Zhong M, Kawaguchi R, Ter-Stepanian M, Kassai M, Sun H. Vitamin A transport and the transmembrane pore in the cell-surface receptor for plasma retinol binding protein. PLoS One. 2013;8(11):e73838. (PubMed)
6. See AW, Clagett-Dame M. The temporal requirement for vitamin A in the developing eye: mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev Biol. 2009;325(1):94-105. (PubMed)
7. Theodosiou M, Laudet V, Schubert M. From carrot to clinic: an overview of the retinoic acid signaling pathway. Cell Mol Life Sci. 2010;67(9):1423-1445. (PubMed)
8. Lefebvre P, Martin PJ, Flajollet S, Dedieu S, Billaut X, Lefebvre B. Transcriptional activities of retinoic acid receptors. Vitam Horm. 2005;70:199-264. (PubMed)
9. Amann PM, Eichmuller SB, Schmidt J, Bazhin AV. Regulation of gene expression by retinoids. Curr Med Chem. 2011;18(9):1405-1412. (PubMed)
10. Pendaries V, Verrecchia F, Michel S, Mauviel A. Retinoic acid receptors interfere with the TGF-beta/Smad signaling pathway in a ligand-specific manner. Oncogene. 2003;22(50):8212-8220. (PubMed)
11. Masia S, Alvarez S, de Lera AR, Barettino D. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21(10):2391-2402. (PubMed)
12. Qiao J, Paul P, Lee S, et al. PI3K/AKT and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochem Biophys Res Commun. 2012;424(3):421-426. (PubMed)
13. Canon E, Cosgaya JM, Scsucova S, Aranda A. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol Biol Cell. 2004;15(12):5583-5592. (PubMed)
14. Kirchmeyer M, Koufany M, Sebillaud S, Netter P, Jouzeau JY, Bianchi A. All-trans retinoic acid suppresses interleukin-6 expression in interleukin-1-stimulated synovial fibroblasts by inhibition of ERK1/2 pathway independently of RAR activation. Arthritis Res Ther. 2008;10(6):R141. (PubMed)
15. Amengual J, Golczak M, Palczewski K, von Lintig J. Lecithin:retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J Biol Chem. 2012;287(29):24216-24227. (PubMed)
16. Noy N. Signaling by retinol and its serum binding protein. Prostaglandins Leukot Essent Fatty Acids. 2014;93:3-7. (PubMed)
17. Berry DC, Jacobs H, Marwarha G, et al. The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J Biol Chem. 2013;288(34):24528-24539. (PubMed)
18. Marwarha G, Berry DC, Croniger CM, Noy N. The retinol esterifying enzyme LRAT supports cell signaling by retinol-binding protein and its receptor STRA6. FASEB J. 2014;28(1):26-34. (PubMed)
19. Farmer SR. Molecular determinants of brown adipocyte formation and function. Genes Dev. 2008;22(10):1269-1275. (PubMed)
20. Kiefer FW, Vernochet C, O'Brien P, et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med. 2012;18(6):918-925. (PubMed)
21. Nallamshetty S, Le PT, Wang H, et al. Retinaldehyde dehydrogenase 1 deficiency inhibits PPARgamma-mediated bone loss and marrow adiposity. Bone. 2014;67:281-291. (PubMed)
22. Kiefer FW, Orasanu G, Nallamshetty S, et al. Retinaldehyde dehydrogenase 1 coordinates hepatic gluconeogenesis and lipid metabolism. Endocrinology. 2012;153(7):3089-3099. (PubMed)
23. Green HN, Mellanby E. Vitamin A as an anti-infective agent. Br Med J. 1928;2(3537):691-696. (PubMed)
24. Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic Acid. J Immunol. 2014;192(7):2953-2958. (PubMed)
25. Spears K, Cheney C, Zerzan J. Low plasma retinol concentrations increase the risk of developing bronchopulmonary dysplasia and long-term respiratory disability in very-low-birth-weight infants. Am J Clin Nutr. 2004;80(6):1589-1594. (PubMed)
26. Barber T, Esteban-Pretel G, Marin MP, Timoneda J. Vitamin A Deficiency and Alterations in the Extracellular Matrix. Nutrients. 2014;6(11):4984-5017. (PubMed)
27. Semba RD, Bloem MW. The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur J Clin Nutr. 2002;56(4):271-281. (PubMed)
28. Allen LH. Iron supplements: scientific issues concerning efficacy and implications for research and programs. J Nutr. 2002;132(4 Suppl):813S-819S. (PubMed)
29. Christian P, West KP, Jr. Interactions between zinc and vitamin A: an update. Am J Clin Nutr. 1998;68(2 Suppl):435S-441S. (PubMed)
30. Auld DS, Bergman T. Medium- and short-chain dehydrogenase/reductase gene and protein families : The role of zinc for alcohol dehydrogenase structure and function. Cell Mol Life Sci. 2008;65(24):3961-3970. (PubMed)
31. Suharno D, West CE, Muhilal, Karyadi D, Hautvast JG. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet. 1993;342(8883):1325-1328. (PubMed)
32. Jang JT, Green JB, Beard JL, Green MH. Kinetic analysis shows that iron deficiency decreases liver vitamin A mobilization in rats. J Nutr. 2000;130(5):1291-1296. (PubMed)
33. Rosales FJ, Jang JT, Pinero DJ, Erikson KM, Beard JL, Ross AC. Iron deficiency in young rats alters the distribution of vitamin A between plasma and liver and between hepatic retinol and retinyl esters. J Nutr. 1999;129(6):1223-1228. (PubMed)
34. Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr. 2011;94(2):658S-665S. (PubMed)
35. Underwood BA, Arthur P. The contribution of vitamin A to public health. Faseb J. 1996;10(9):1040-1048. (PubMed)
36. Solomons NW. Vitamin A. In: Erdman JJ, Macdonald I, Zeisel S, eds. Present Knowledge in Nutrition. 10th ed: John Wiley & Sons, Ltd.; 2012:149-184.
37. Sherwin JC, Reacher MH, Dean WH, Ngondi J. Epidemiology of vitamin A deficiency and xerophthalmia in at-risk populations. Trans R Soc Trop Med Hyg. 2012;106(4):205-214. (PubMed)
38. World Health Organization. Guideline - Vitamin A supplementation for infants and children 6-59 months of age - Guideline. Geneva 2011.
39. World Health Organization. Guideline - Neonatal vitamin A supplementation Geneva 2011.
40. World Health Organization. Guideline - Vitamin A supplementation for infants 1–5 months of age - Guideline. Geneva 2011.
41. Gilbert C, Awan H. Blindness in children. BMJ. 2003;327(7418):760-761. (PubMed)
42. Semba RD. Vitamin A and human immunodeficiency virus infection. Proc Nutr Soc. 1997;56(1B):459-469. (PubMed)
43. Field CJ, Johnson IR, Schley PD. Nutrients and their role in host resistance to infection. J Leukoc Biol. 2002;71(1):16-32. (PubMed)
44. WHO, UNICEF, IVACG Task Force. Vitamin A supplements: a guide to their use in the treatment and prevention of vitamin A deficiency and xerophthalmia. Geneva: World Health Organization; 1997.
45. Thornton KA, Mora-Plazas M, Marin C, Villamor E. Vitamin A deficiency is associated with gastrointestinal and respiratory morbidity in school-age children. J Nutr. 2014;144(4):496-503. (PubMed)
46. Wiysonge CS, Shey M, Kongnyuy EJ, Sterne JA, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2011(1):CD003648. (PubMed)
47. Semba RD, Miotti PG, Chiphangwi JD, et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. Lancet. 1994;343(8913):1593-1597. (PubMed)
48. World Health Organization. Guideline - Vitamin A supplementation in pregnancy for reducing the risk of mother-to-child transmission of HIV. Geneva 2011.
49. Zimmermann MB, Adou P, Torresani T, Zeder C, Hurrell RF. Effect of oral iodized oil on thyroid size and thyroid hormone metabolism in children with concurrent selenium and iodine deficiency. Eur J Clin Nutr. 2000;54(3):209-213. (PubMed)
50. Zimmermann MB, Wegmuller R, Zeder C, Chaouki N, Torresani T. The effects of vitamin A deficiency and vitamin A supplementation on thyroid function in goitrous children. J Clin Endocrinol Metab. 2004;89(11):5441-5447. (PubMed)
51. Zimmermann MB. Interactions of vitamin A and iodine deficiencies: effects on the pituitary-thyroid axis. Int J Vitam Nutr Res. 2007;77(3):236-240. (PubMed)
52. Zimmermann MB, Jooste PL, Mabapa NS, et al. Vitamin A supplementation in iodine-deficient African children decreases thyrotropin stimulation of the thyroid and reduces the goiter rate. Am J Clin Nutr. 2007;86(4):1040-1044. (PubMed)
53. Maronn M, Allen DM, Esterly NB. Phrynoderma: a manifestation of vitamin A deficiency?... The rest of the story. Pediatr Dermatol. 2005;22(1):60-63. (PubMed)
54. Herring W, Nowicki MJ, Jones JK. An uncommon cause of esophagitis. Answer to the clinical challenges and images in GI question: image 1: esophageal hyperkeratosis secondary to vitamin A deficiency. Gastroenterology. 2010;139(2):e6-7. (PubMed)
55. Weber D, Grune T. The contribution of beta-carotene to vitamin A supply of humans. Mol Nutr Food Res. 2012;56(2):251-258. (PubMed)
56. Food and Nutrition Board, Institute of Medicine. Vitamin A. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, D.C.: National Academy Press; 2001:65-126. (National Academy Press)
57. Ravishankar C, Nafday S, Green RS, et al. A trial of vitamin A therapy to facilitate ductal closure in premature infants. J Pediatr. 2003;143(5):644-648. (PubMed)
58. Tyson JE, Wright LL, Oh W, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999;340(25):1962-1968. (PubMed)
59. Wardle SP, Hughes A, Chen S, Shaw NJ. Randomised controlled trial of oral vitamin A supplementation in preterm infants to prevent chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2001;84(1):F9-F13. (PubMed)
60. Ambalavanan N, Kennedy K, Tyson J, Carlo WA. Survey of vitamin A supplementation for extremely-low-birth-weight infants: is clinical practice consistent with the evidence? J Pediatr. 2004;145(3):304-307. (PubMed)
61. Laughon MM. Vitamin A shortage and risk of bronchopulmonary dysplasia. JAMA Pediatr. 2014;168(11):995-996. (PubMed)
62. Tolia VN, Murthy K, McKinley PS, Bennett MM, Clark RH. The effect of the national shortage of vitamin A on death or chronic lung disease in extremely low-birth-weight infants. JAMA Pediatr. 2014;168(11):1039-1044. (PubMed)
63. Gadhia MM, Cutter GR, Abman SH, Kinsella JP. Effects of early inhaled nitric oxide therapy and vitamin A supplementation on the risk for bronchopulmonary dysplasia in premature newborns with respiratory failure. J Pediatr. 2014;164(4):744-748. (PubMed)
64. Meyer S, Gortner L, NeoVita ATI. Early postnatal additional high-dose oral vitamin A supplementation versus placebo for 28 days for preventing bronchopulmonary dysplasia or death in extremely low birth weight infants. Neonatology. 2014;105(3):182-188. (PubMed)
65. Babu TA, Sharmila V. Vitamin A supplementation in late pregnancy can decrease the incidence of bronchopulmonary dysplasia in newborns. J Matern Fetal Neonatal Med. 2010;23(12):1468-1469. (PubMed)
66. Thorne-Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26 Suppl 1:36-54. (PubMed)
67. Imdad A, Yakoob MY, Sudfeld C, Haider BA, Black RE, Bhutta ZA. Impact of vitamin A supplementation on infant and childhood mortality. BMC Public Health. 2011;11 Suppl 3:S20. (PubMed)
68. Gogia S, Sachdev HS. Vitamin A supplementation for the prevention of morbidity and mortality in infants six months of age or less. Cochrane Database Syst Rev. 2011(10):CD007480. (PubMed)
69. Fisker AB, Bale C, Rodrigues A, et al. High-dose vitamin A with vaccination after 6 months of age: a randomized trial. Pediatrics. 2014;134(3):e739-748. (PubMed)
70. Aaby P, Martins CL, Garly ML, et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ. 2010;341:c6495. (PubMed)
71. Benn CS, Martins CL, Fisker AB, et al. Interaction between neonatal vitamin A supplementation and timing of measles vaccination: a retrospective analysis of three randomized trials from Guinea-Bissau. Vaccine. 2014;32(42):5468-5474. (PubMed)
72. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):CD001479. (PubMed)
73. American Academy of Pediatrics Committee on Infectious Diseases: Vitamin A treatment of measles. Pediatrics. 1993;91(5):1014-1015. (PubMed)
74. Bello S, Meremikwu MM, Ejemot-Nwadiaro RI, Oduwole O. Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. Cochrane Database Syst Rev. 2014;1:CD007719. (PubMed)
75. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150-1155. (PubMed)
76. Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743-1750. (PubMed)
77. Palozza P, Simone R, Mele MC. Interplay of carotenoids with cigarette smoking: implications in lung cancer. Curr Med Chem. 2008;15(9):844-854. (PubMed)
78. Cheng TY, Goodman GE, Thornquist MD, et al. Estimated intake of vitamin D and its interaction with vitamin A on lung cancer risk among smokers. Int J Cancer. 2014;135(9):2135-2145. (PubMed)
79. Cortes-Jofre M, Rueda JR, Corsini-Munoz G, Fonseca-Cortes C, Caraballoso M, Bonfill Cosp X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2012;10:CD002141. (PubMed)
80. Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111-121. (PubMed)
81. Booij MT, Van De Kerkhof PC. Acitretin revisited in the era of biologics. J Dermatolog Treat. 2011;22(2):86-89. (PubMed)
82. Orfanos CE, Zouboulis CC. Oral retinoids in the treatment of seborrhoea and acne. Dermatology. 1998;196(1):140-147. (PubMed)
83. Thielitz A, Gollnick H. Topical retinoids in acne vulgaris: update on efficacy and safety. Am J Clin Dermatol. 2008;9(6):369-381. (PubMed)
84. Vishwanathan R, Johnson EJ. Eye disease. In: Erdman JJ, Macdonald I, Zeisel S, eds. Present Knowledge in Nutrition. 10th ed: John Wiley & Sons, Ltd; 2012:939-981.
85. Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795-1809. (PubMed)
86. Berson EL, Rosner B, Sandberg MA, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(6):761-772. (PubMed)
87. Sibulesky L, Hayes KC, Pronczuk A, Weigel-DiFranco C, Rosner B, Berson EL. Safety of <7500 RE (<25000 IU) vitamin A daily in adults with retinitis pigmentosa. Am J Clin Nutr. 1999;69(4):656-663. (PubMed)
88. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. 2nd edition ed: Thomson Reuters; 2008.
89. Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83(2):191-201. (PubMed)
90. Russell RM. The vitamin A spectrum: from deficiency to toxicity. Am J Clin Nutr. 2000;71(4):878-884. (PubMed)
91. World Health Organization. Guideline - Vitamin A supplementation in pregnant women. Geneva 2011.
92. Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57(6):665-667. (PubMed)
93. Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003;348(4):287-294. (PubMed)
94. Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Retinol intake and bone mineral density in the elderly: the Rancho Bernardo Study. J Bone Miner Res. 2002;17(8):1349-1358. (PubMed)
95. Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA. 2002;287(1):47-54. (PubMed)
96. Rejnmark L, Vestergaard P, Charles P, et al. No effect of vitamin A intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos Int. 2004;15(11):872-880. (PubMed)
97. Sowers MF, Wallace RB. Retinol, supplemental vitamin A and bone status. J Clin Epidemiol. 1990;43(7):693-699. (PubMed)
98. Ballew C, Galuska D, Gillespie C. High serum retinyl esters are not associated with reduced bone mineral density in the Third National Health And Nutrition Examination Survey, 1988-1994. J Bone Miner Res. 2001;16(12):2306-2312. (PubMed)
99. Wu AM, Huang CQ, Lin ZK, et al. The relationship between vitamin A and risk of fracture: meta-analysis of prospective studies. J Bone Miner Res. 2014;29(9):2032-2039. (PubMed)
100. Conaway HH, Henning P, Lerner UH. Vitamin a metabolism, action, and role in skeletal homeostasis. Endocr Rev. 2013;34(6):766-797. (PubMed)
101. Johansson S, Melhus H. Vitamin A antagonizes calcium response to vitamin D in man. J Bone Miner Res. 2001;16(10):1899-1905. (PubMed)
102. Caire-Juvera G, Ritenbaugh C, Wactawski-Wende J, Snetselaar LG, Chen Z. Vitamin A and retinol intakes and the risk of fractures among participants of the Women's Health Initiative Observational Study. Am J Clin Nutr. 2009;89(1):323-330. (PubMed)
103. Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003;27(3):220-231. (PubMed)
Can Vitamin C Increase Appetite
Source: https://lpi.oregonstate.edu/mic/vitamins/vitamin-A


Tidak ada komentar:
Posting Komentar